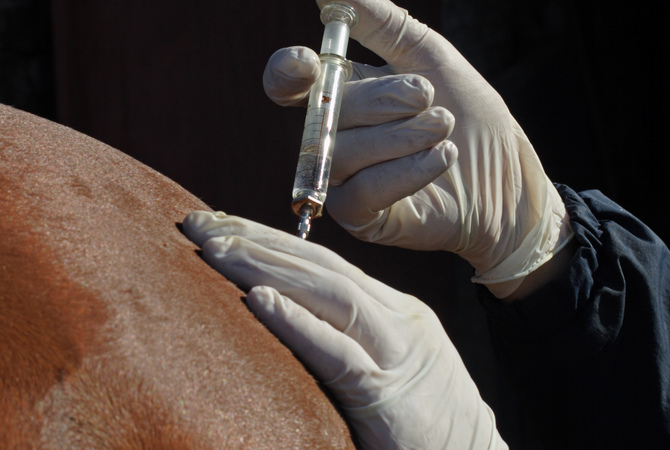
In November 2019, Zimeta was approved by the U.S. Food and Drug Administration Center for Veterinary Medicine for the control of pyrexia (fever) in horses. Recently, both the United States Equestrian Federation (USEF) and American Quarter Horse Association (AQHA) updated their policies to provide competing members with guidelines for the use of Zimeta.

A digital, infrared thermometer for taking a horse's temperature
Guidelines for using Zimeta™ (dipyrone injection) in competitive horses affects competing members use of the first and only FDA-approved product for control of fever in horses.
© 2019 by BAFX Products
For both organizations, use of Zimeta will require a properly filed medication report form documenting a 24-hour withdrawal. Additionally, administration of Zimeta will not constitute the use of a second non-steroidal anti-inflammatory drug (NSAID), which is prohibited by USEF and AQHA rules, respectively.1,2
Fever is a clinical sign commonly associated with various underlying infectious and non-infectious diseases in the horse, explains Daniel Dreyfuss, DVM, MA, veterinary science liaison at Kindred Biosciences, Inc. (NASDAQ: KIN).
“For equine athletes, fever is an important sign. Fever is a horse’s response to infectious or inflammatory processes. It is important that potentially infectious horses either not travel to the competition or follow appropriate biosecurity protocols if at the competition venue,” Dreyfuss says. “Following the USEF and AQHA policies gives owners and veterinarians flexibility to use the only NSAID that is FDA-approved to control fever in horses.”
KindredBio is working with the USEF, AQHA, and other equine organizations, to provide the necessary information to determine adequate withdrawal times for Zimeta. This is the first time dipyrone has been FDA approved for veterinary use in the United States.
Dipyrone, also known as metamizole, is approved and widely used in many other countries. The Fédération Equestre Internationale (FEI) has established the detection time for dipyrone at 72 hours, which is the shortest established time among NSAIDs approved in the United States.3
For additional questions about the USEF and AQHA policies, please contact the USEF Equine Drugs and Medications Program at 1-800-633-2472 or visit aqha.com. For questions about Zimeta, contact KindredBio Customer Care at 1-888-608-2542.
Zimeta is indicated for the control of pyrexia (fever) in horses
Important Safety Information
Zimeta™ (dipyrone injection) should not be used more frequently than every 12 hours. For use in horses only. Do not use in horses with a hypersensitivity to dipyrone, horses intended for human consumption or any food producing animals, including lactating dairy animals. Not for use in humans, avoid contact with skin and keep out of reach of children. Take care to avoid accidental self-injection and use routine precautions when handling and using loaded syringes. Prior to use, horses should undergo a thorough history and physical examination by a veterinarian. Monitor for signs of abnormal bleeding and use caution in horses at risk for hemorrhage. Concurrent use with other NSAIDs, corticosteroids and drugs associated with kidney toxicity, should be avoided. As a class, NSAIDs may be associated with gastrointestinal, kidney, and liver toxicity. The most common adverse reactions observed during clinical trials were elevated glucose conversion enzymes, decreased blood protein, and gastric ulcers. For product label, including complete safety information, visit kindredbio.com/Zimeta-pi.
About Kindred Biosciences
Kindred Biosciences is a commercial-stage biopharmaceutical company focused on saving and improving the lives of horses and companion animals. Its mission is to bring to horses and pets the same kinds of safe and effective medicines that human family members enjoy. The company’s strategy is to identify compounds and targets that have already demonstrated safety and efficacy in humans and to develop therapeutics based on these validated compounds and targets for cats, dogs, and horses. The company has a deep pipeline of novel drugs and biologics in development across many therapeutic classes.
Visit http://kindredbio.com for more information.
References
1 United States Equestrian Federation. US Equestrian Announces Requirements for Use of FDA-Approved Zimeta™ (Dipyrone Injection). Dec. 31, 2019. Available at: https://www.usef.org/media/press-releases/us-equestrian-announces-requirements-for-use-of. [Access Date: Jan. 28, 2020].
2 American Quarter Horse Association. Zimeta Approved by AQHA as a Therapeutic Medication. January 28, 2020. Available at: https://www.aqha.com/-/zimeta-approved-by-aqha-as-a-therapeutic-medication. [Access Date: Jan. 28, 2020].
3 Fédération Equestre Internationale. FEI List of Detection Times. July 13, 2018. Available from: https://inside.fei.org/system/files/FEI%20Detection%20Times%202018_0.pdf. [Access Date: Jan. 28, 2020].
Press release provided by Beckie Peskin - marketing@kindredbio.com